A few weeks ago I talked about an innovative applied research experiment being done aboard the International Space Station for Eli Lilly. They are interested in the process by which tablets dissolve, since this can be a problem for helping patients get the dose of medicine they need. Because microgravity allows study of diffusion without buoyancy or density-driven convection, these processes can be slower, allowing for better visualization and mathematical modeling.
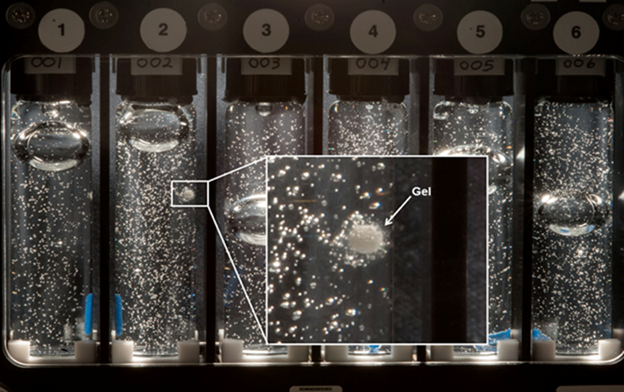
The PIs of this experiment have allowed us to share the early visual results from their ISS experiment. In the image above, you can see an example of a significant gel interface that formed between the tablet and the solution which was not observed to the same extent on Earth. The ground controls are pending, but based on preliminary results, the rate of dissolution was significantly longer in the microgravity experiment, an unexpected and interesting result.
In chemistry, wetting refers to spreading of a liquid over a solid material’s surface, and is a key aspect of the material’s ability to dissolve. This investigation studies how certain materials used in the pharmaceutical industry dissolve in water while in microgravity. Results from this investigation could help improve the design of tablets that dissolve in the body to deliver drugs, thereby improving drug design for medicines used in space and on Earth.

