In today’s A Lab Aloft, Joshua S. Alwood, Ph.D., shares his postdoctoral research into the impact of microgravity and ionizing radiation exposure on bone health – work that led to his receiving the 2012 Presidential Early Career Award for Scientists and Engineers.
I am honored to be a recipient of a 2012 Presidential Early Career Award for Scientists and Engineers (PECASE). This award is based on the cumulative body of work achieved during my postdoctoral fellowship at NASA’s Ames Research Center in Moffett Field, Calif., while working with my advisor Ruth Globus, Ph.D. and on my contribution to experiments that flew on International Space Station assembly flights STS-131 and STS-135 with collaborator Eduardo Almeida, Ph.D.

The big question motivating our studies is, how does spaceflight cause bone loss in astronauts? While weightless, astronauts lose about one percent bone mineral density per month. To put this into context, this is about 10 times faster than osteoporosis typically progresses on Earth. The longer the mission, particularly outside Earth’s protective magnetic fields, the greater the doses of ionizing radiation astronauts will accumulate, which may also negatively impact the skeleton.

In our ground-based research, we asked the following questions: how do the conditions of simulated weightlessness and ionizing radiation exposure affect the skeleton, and are the results any different when these conditions are combined? Our results suggest that each condition causes bone loss on its own, though at differing rates and severity. Together, weightlessness and radiation exposure cause bone loss to worsen beyond either treatment alone. These results have to do with the behavior of different types of bone cells. Combined, the balance of bone formation and bone resorption—breaking down—define a normal process called bone remodeling, which the body uses to maintain a healthy skeletal structure throughout life.
Following radiation exposure, there is a rapid stimulation of bone resorption—this can lead to a net loss of skeletal tissue caused by a specific cell called an osteoclast. We quantify this by measuring the number of osteoclast cells on the surface on the bone and their level of activity through specific proteins secreted into circulation. At the tissue level, we use X-ray imaging and computed tomography to build and quantify three-dimensional models of the skeleton. This rapid resorption is concentrated, yet it appears to be short-lived. At the doses we’ve investigated, it doesn’t get worse after the initial burst.
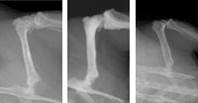
In contrast, structural changes caused by simulated weightlessness gradually appear. Simulated weightlessness activates bone resorption by the osteoclasts and additionally reduces bone formation by inhibiting a second cell type called an osteoblast.
Exposure to space radiation affects osteoblasts as well. Our research shows that at the estimated doses potentially accumulated over a three-year Mars mission, long-lasting effects to the osteoblast-lineage cells occur. This may result in abnormal bone remodeling in the long-term. To this end, we determined that spongy bone—found on the inside of some bones—recovered less efficiently following radiation exposure vs. recovery from simulated microgravity.
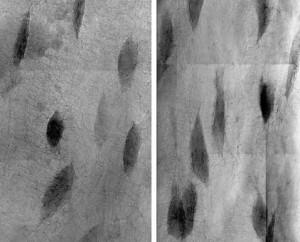
The second component of the PECASE acknowledges my work with the Biospecimen Sharing Program for shuttle flight STS-131. Along with Almeida, the project investigator, I used a new application of a high-resolution (30 nanometer) X-ray transmission microscope at the SLAC National Accelerator Laboratory to analyze bone health following 15 days of spaceflight.
We scanned the bones and processed the images for three-dimensional tomographic analyses. As a result, we were able to quantify changes in the bone’s osteocyte cells. In a unique mechanism of bone loss, osteocytes actively remodel and enlarge their living spaces, known as lacunae, in response to spaceflight. This is an additional mode of bone resorption that occurs during spaceflight at the bone’s surface.
My research employs a basic biology approach with the overriding motivation to enable human exploration of space. In other words, the goal is to expand the envelope of mission durations, the distance from Earth that missions can access, and to mitigate the skeletal consequences following radiation exposure for astronauts. My colleagues and I use a basic approach to uncover the cellular and molecular mechanisms that underlie skeletal changes in the space environment. Eventually we can use this knowledge to develop countermeasures to better manage astronaut skeletal health.
On Earth, this research has applications towards improving our knowledge of bone diseases such as osteoporosis. It may also help people living sedentary lifestyles, providing positive impacts to their health. The sedentary environment is somewhat similar to weightlessness. The take-home message: use your skeleton or lose it. The ability to work on advancing an area of biology that may help humans both in space and on the ground is truly its own reward.
I was inspired to enter this area of research from childhood experiences. I grew up in Florida and witnessed space shuttle launches in my front yard. This really captivated my attention towards space. As I got older, I learned about the skeleton and how it is a living structure that adapts to its mechanical environment. For example, joggers experience forces equivalent to about three times their body weight. Those two factors overlapped in my extracurricular readings on astronauts and the changes in their bodies during weightlessness. That connection propelled me to focus on science and engineering in my education, and further to study the skeleton. I am driven to continually learn new things, which prompted me to enter graduate school and become an independent researcher.

Although I do not have an immediate investigation going to the space station, I am applying for opportunities through NASA’s Space Biology Project. It’s an exciting time to be part of NASA’s research program. In the meantime, I continue to develop hypotheses worth studying aboard the space station and generating preliminary evidence while working in my lab on the ground. My goal is to eventually take my science into orbit or beyond.
The work cited in this PECASE Award was made possible by key funding organizations, including NASA Space Biology; grants to Globus from the National Space Biomedicine Research Institute; the U.S. Department of Energy and the NASA Space Radiation Project Element; and grants to Almeida from the NASA Bion-M1 Biospecimen Sharing Program and the National Institutes of Health / National Institute of Biomedical Imaging and Bioengineering.

Joshua S. Alwood, Ph.D., is a senior scientist with CSS-Dynamac working at the Bone and Signaling Lab at NASA’s Ames Research Center in Moffett Field, Calif. Alwood earned his Ph.D. in Aeronautics and Astronautics from Stanford University, as well B.S. degrees in Physics and Astronomy from the University of Florida.
