In today’s A Lab Aloft, guest blogger Donald Barker explains the complex world of colloids and how studying them aboard the International Space Station helps us understand and use them better here on Earth.
Colloids are fascinating. They are part of our daily lives, found in everything from our bodies to the products we purchase at the convenience store. Manufacturers use colloids and their unique structure and properties for wine making, coloring glass, and fabric softeners. You will even find them in your daily glass of milk!
So what exactly is a colloid? In our daily lives we generally think of traditional forms of matter: solids, liquids, gasses. Colloids, however, exist around and near the boundaries of these states—not quite being one or the other. Colloids generally take one of the following forms: aerosols, emulsions, gels, sols, foams or films.
Colloids form when particles disperse throughout a solvent, usually a liquid, depending on the purpose of the mixture. Colloidal particles are too small to be seen with ordinary optical microscopes. The size of the particles is somewhere between atoms and molecules, roughly 10 to 1,000 nanometer (or 1 micrometer). At such minute scales, physical interactions seem to work in mysterious and magical ways. This critical particulate size range is exactly where it needs to be in order to make it unlikely that they will settle out of their mixture; this property is why they are so useful.
For researchers interested in colloids, the International Space Station provides a unique laboratory environment to examine their properties. On Earth, gravity-induced settling or sedimentation changes or destroys the structure of a colloid over time. In microgravity, scientists have a stable setting where they can observe the particle interactions and structures while changing various environmental parameters, such as temperature and pressure.
Researchers are directly interested in the interactions occurring between the surface of the colloidal particles and their solvent. The mixture behaves in different ways, based on both the size of the colloid particles and their interactions with the solvent. Ongoing colloid investigations make use of space station facilities like the Microgravity Science Glovebox (MSG), the Fluids Integrated Rack (FIR) and the Light Microscopy Module (LMM).

Don Pettit, Expedition 30 Flight Engineer, working with the Microgravity Sciences Glovebox (MSG) in the U.S. Laboratory. (Credit: NASA)
Understanding the behavior of colloids allows scientists to create models and process that can be used to enhance food and chemical preservation, evenly distribute ingredients used to produce glues, jellies and gelatins or even to control the movement of light in optical devices and materials. Controlling colloidal mixtures can help global industries create better, more reliable products and processes.

Astronaut T.J. Creamer working at the Light Microscopy Module, or LMM, facility aboard the International Space Station. (Credit: NASA)
Colloid studies occur regularly on the space station and one of these investigations is the Advanced Colloids Experiment-1, or ACE-1. The ACE-1 containment device holds up to 20 sample disks that, in turn, each hold up to 10 wells of colloidal particles. Astronauts mix the samples in each disk and then observe them using the LMM. The crew member takes pictures for downlink to investigators for analysis on the ground, where the investigators monitor and record colloidal structural changes and particle interactions.
The goal of ACE-1 is to understand how colloids move over time in the microgravity environment. By seeing how these particles naturally aggregate or cluster without the pull of gravity, scientists can learn how to control them. Essentially, they are looking to see how nature grows at the particle level, forming order out of disorder. Researchers hope to see how well their theoretical understanding compares to the world of everyday observations.
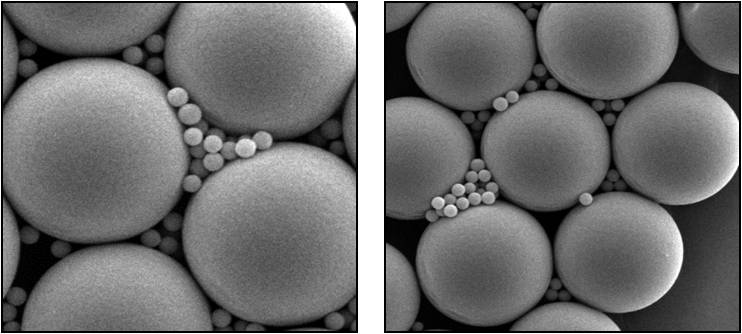
Scanning electron microscope, or SEM, images of a mixture of 3.8 micron diameter “seed” particles together with the bulk colloid—0.33 micron diameter Polymethylmetachrylate, or PMMA, spheres. Recent International Space Station colloid studies show a cycle of replication, as large crystals generate smaller ones that separate and continue to grow and produce. (Credit: P.M. Chaikin and A.D. Hollingsworth, New York University)
Another set of colloid studies aboard station is the Binary Colloidal Alloy/Aggregation Test, or BCAT investigation. The BCAT-6 study is the latest in a series of related experiments run on the station. It uses a sample growth module that holds 10 couvettes—small test tubes, each with a different colloid solution mixture. Observations begin following the stirring of each sample. Manual and automated time-lapse photographs record the separation over time.
Objectives of the BCAT suite of investigations include studying the dynamics between phase separation and crystallization in the solution, as well as how order arises out of disorder in microgravity.
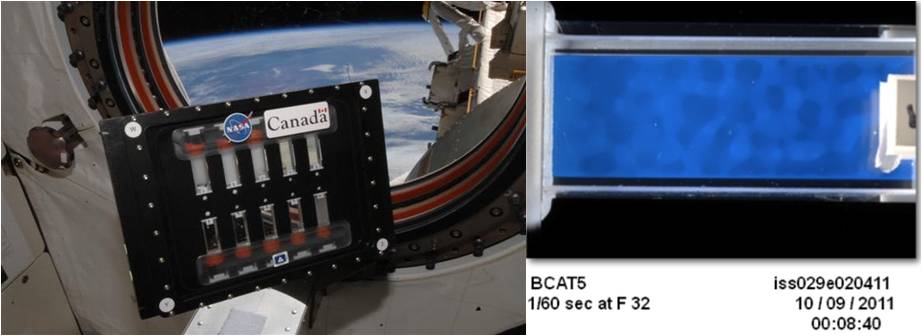
These images show the BCAT sample growth module (left) and a close up of a BCAT-5 sample (right) showing structural changes in the mixture aboard the International Space Station. (Credit: NASA)
Another station investigation is the Selectable Optical Diagnostics Instrument – Aggregation of Colloidal Suspensions, or SODI-Colloid. This is a series of experiments using cell chambers that hold individual samples that are measured optically using a Near-Field Scattering (NFS) technique within the MSG.
Understanding how the particles making up colloids react, move, arrange and form crystals as the temperature reaches the critical point can help with the development of materials for devices using electromagnetic waves and signals to manipulate optics, such as plasma TVs.
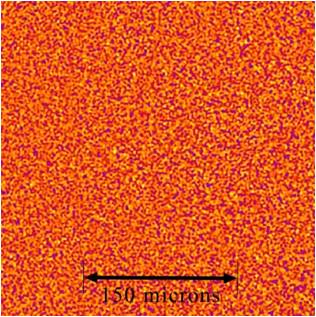
This image shows a false color NFS image during aggregation showing the distribution of particles on the smallest of scales. (Credit: S. Mazzoni, ESA)
A very different colloidal mixture—a magnetic one—is studied in Investigating the Structure of Paramagnetic Aggregates from Colloidal Emulsions-3, or InSPACE-3. This series of microgravity studies focuses on mixtures with magnetizable particles of varying shape (spheres to ellipsoids) exposed to an alternating magnetic field.
These kinds of fluids are considered to be “smart” materials, which transition into a solid-like state or gel when exposed to a magnetic field. Understanding how to control and produce colloidal materials of this kind may help in the engineering of vibration dampening systems, enhanced earthquake structural designs, robotic systems, tunable dampers, and brake and clutch systems.
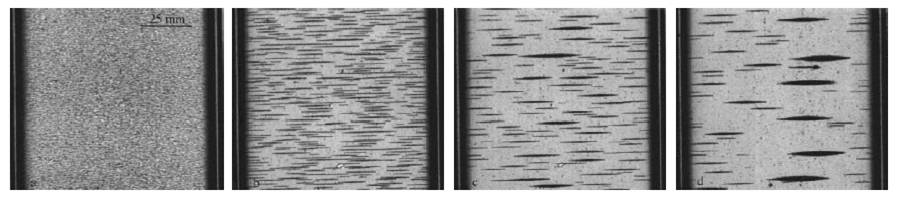
This image shows the evolution of colloidal structure within an applied alternating magnetic field. (Credit: N. Hall, NASA)
On Earth, colloids tend to collapse, change form, or sink, depending on particle size, shape, composition, fluid solvent mixture or environmental conditions; all highly dependent on the effects of gravity. This is why the space station provides an ideal laboratory setting for researchers to tease out the underlying physical properties of colloidal solutions. As we better understand the special and fascinating properties of colloids, researchers will be able to devise better technologies and products for use back here on Earth.

Donald C. Barker (Credit: NASA)
Donald C. Barker is a scientist with the International Space Station Program Science Office. Previously Barker served as a lead systems engineer, flight controller and researcher at the Johnson Space Center. He holds a double Bachelor of Science degree in Physics and Psychology from Colorado State University, Master of Science degrees in Physics, Psychology, Mathematics and Space Architecture, and he is currently pursuing a Doctor in Philosophy in Planetary Geology at the University of Houston.

Thanks, Don, for writing about such tangible benefits of space station research. So much of what we hear about in the news from ISS focuses on overcoming the effects of living in space, which is important but repetitive. You’re talking about things the promise of which got this program funded, and should keep it flying. This kind of discovery is how we will create many of the new jobs, products, and whole industries of the future. Nice work, and please keep educating the naysayers in Congress!