In today’s A Lab Aloft, Mike Hicks, project scientist at NASA’s Glenn Research Center in Cleveland, blogs about discovering the “cool” world of combustion aboard the International Space Station.
In a recent posting on this blog one of our International Space Station combustion researchers, Sandra Olson, noted that when it comes to combustion experiments in microgravity one should expect surprises. This has certainly proven to be true with one of our liquid fuel combustion investigations currently operating aboard the space station.
The Flammability and Extinction (FLEX) study burns liquid fuels dispensed in the form of small, single droplets. The goal is to answer two key questions: the first is how difficult it is to keep a flame burning in microgravity? We call this flammability mapping. The second is how effective is gaseous carbon dioxide (CO2) (or other diluents) in extinguishing spacecraft fires? Gaseous CO2 is particularly important because this is the current fire suppressant used in the U.S. module of the space station.
To answer these and other “burning questions” the FLEX team of scientists studies small droplets of fuel. This approach allows a large number of tests to take place under a wide range of conditions. The flame that results from igniting these fuel droplets (ranging in size from 1.0 mm to 6.0 mm in diameter) achieves a shape that can only happen in a reduced gravity environment—that is, they become little glowing balls of fire.
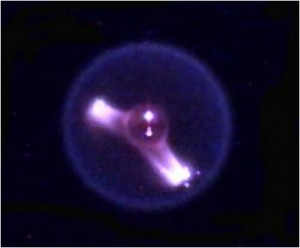
Within fractions of a second following ignition, the reaction front—visualized as a flame—quickly wraps around the fuel droplet and assumes a spherical shape. This remains mostly motionless until the droplet of fuel is consumed or until the conditions are such that the flame can no longer exist. You can watch the Strange Flames on the International Space Station video to see these microgravity flames in action.
The overarching goal of FLEX is to better understand the complicated physical processes behind determining whether or not a flame can exist in a given environment for a given fuel type. The environment varies based on the amount of oxygen in the combustion chamber along with other gases that balance out the test atmosphere.
Since combustion processes are rich in physics and chemistry, there are many parameters that play a role in flame survival. The FLEX experiments allow scientists a way to study these competing physical processes—such as the rate of fuel consumption, fuel characteristics, the controlling energy and mass transfer processes, and the physical properties of the gases that make up the test environment. By understanding and modeling these processes, we hope to better predict fire behavior in space. This also leads to Earth technology advances, such as more fuel efficient engine designs.
FLEX recently led us to a “cool” discovery, igniting the imaginations of many combustion researchers. During one of their many late night test sessions, the FLEX operations team noticed a very peculiar orange afterglow about 20 seconds after the flame had extinguished. It was so intense that the team first thought something else other than the fuel droplet was burning.

After a few repeated tests, the team concluded that this bright afterglow resulted from tiny droplets of re-condensed fuel vapor. The idea was that these tiny droplets formed a fog, scattering the yellow-orange backlight that is used to project the droplet’s shadow onto one of the diagnostic cameras. The afterglow was initially dismissed as an artifact of the test configuration—or so we thought.
A few days later we recovered the data from the space station and processed the images for accurate measurements. As we looked closely at the droplet shadow images we realized this glow was actually a clue to something new.
Typically when a flame extinguishes, the evaporation of the fuel droplet will nearly stop, and the droplet stops shrinking, which makes entirely good sense—no flame, well then, no fuel consumption. We discovered with FLEX that under certain conditions when the flame disappeared, the droplet of fuel continued to evaporate at almost the same rate as when the flame was visible. In many cases this “apparent” fuel consumption without a flame lasted longer than with the flame.
Even more startling, we saw that when the pressure of the chamber was slightly increased, the flame momentarily reappeared in a brief flash before quickly going out again. In some cases this phenomena repeated until the fuel was consumed. This finding has a number of interesting ramifications, both from a purely scientific perspective as well as from a fire safety perspective. It provides the first direct observation of a long held concern—the possibility that microgravity conditions were conducive to re-ignition of flammable mixtures.

At first glance, it seemed irrational that the fuel droplet continued to evaporate with no visible flame. Some of the science team argued, myself included, that this could be explained by characteristics of the test chamber environment. It was suggested that once the visible flame extinguished, that there was still a sufficient amount of energy remaining in the surrounding gases to continue the rate of the droplet’s evaporation.
Since buoyant forces are absent in microgravity flames, the hot gases simply remain stationary around the droplet—so we theorized that as these gases cooled, heat transferred back into the droplet. We quickly realized, however, that this would have resulted in a gradual slowing of the droplet’s evaporation rate, which was not the case. During the “dark burning periods” when no flame was visible, the evaporation rates were essentially constant, similar to what is seen with a visible flame. Interestingly, these evaporation rates suddenly stopped when the afterglow of the scattered light began to appear.
In 65 years of previous droplet experiments, and employing a staggering breadth of microgravity test configurations—from converted mine shafts in Japan to experiments performed on the recently retired space shuttles—this phenomena had never been observed. The prior universal observation was that when the flame went out, the rapid evaporation of the droplet stopped. However, with these tests we now had a fuel droplet that continued to be consumed at a rapid rate without an apparent flame.

After a great deal of deliberation, the collective wisdom of the FLEX team came up with a theoretical explanation: “cool flames!” The technical explanation for this phenomena first appeared in Combustion and Flame in an article entitled “Can Cool Flames Support Quasi-Steady Alkane Droplet Burning?”
So what exactly is a cool flame and why is it important? The discovery of cool flames is often attributed to Sir Humphry Davy in 1812. While investigating flames for the purpose of designing safety lamps for coal miners, Davy observed that he could generate flames in the lab that were so weak that they could would not even ignite a match. This finding was followed by W.H. Perkins, who published in 1882 the first investigation of cool flames. Perkins observed that vapors of organic fuels produced what he characterized as “blue lambent flames.” Generally speaking, cool flames are the visible light of oxidation reactions occurring at low temperatures, sometimes nearly 10 times less than normal flame temperatures.

Cool flame temperatures depend on fuel type and atmosphere. For n-heptane—the alkane fuel used in the FLEX experiments—cool flames appear at temperatures just above 600 degrees Fahrenheit. This is about 176 degrees Fahrenheit warmer than the temperature for baking bread and considerably less, by about 1,300 degrees Fahrenheit, than the flame one might roast a marshmallow over. Hence the name “cool flame.”
Despite the name, these flames’ temperatures are still sufficiently high to sustain chemical reactions. Understanding exactly what kind of chemical reactions are taking place, as well as the conditions necessary to propel the chemistry into the high temperature realm of hot flames, are key research areas in the study of cool flames.
Prior to the FLEX discovery, it was thought cool flames were primarily pre-ignition phenomena generally limited to pre-mixed gases. If you’ve ever taken your car in for engine knocking, you’ve experienced an example of this. Pockets of gas in the cylinder undergo low temperature reactions (i.e., cool flames) that result in poorly timed pressure peaks. These deviations from the optimal peak pressure timing that should occur shortly after spark ignition result in the noise often associated with engine knock.
The FLEX study, however, showed a post-ignition cool flame. This occurred in a controlled system where reactants are initially separated, requiring time to travel to the reaction zone, which we see as the flame. This appears as a relatively steady phenomenon, suggesting a delicate balance between heat generated by the reaction and heat losses to the surrounding—to date, only achievable in space.
The microgravity cool flame discovery is significant for a number of reasons. Low temperature combustion in internal combustion engines, for instance, offers a number of advantages: reduced emissions, less wear on engine parts and increased fuel efficiencies. A better understanding of these low temperature burning regimes may help address challenges facing new internal combustion technologies, such as the Homogenous Charge Compression Ignition (HCCI) and Reactivity Charge Compression Ignition (RCCI) engines. Since these technologies lack a spark for localized ignition, knowledge of cool flames may help with precise timing of the combustion event—based, in this case, on a reactant mixture that may only be partially pre-mixed.
Microgravity cool flames are exciting in their own right, because of the potential for refining our understanding of a very complicated combustion phenomena. This is true of all serendipitous discoveries, giving one more example of how the space station provides an opportunity for discoveries that would otherwise never get made.

Mike Hicks, project scientist at NASA’s Glenn Research Center in Cleveland, has been with NASA for 22 years and is currently probing the mysteries of droplet combustion as a co-investigator on the Flammability and Extinction (FLEX) study. He is also the principal investigator on the Supercritical Water Mixture (SCWM) International Space Station flight investigation, which is designed to study precipitation phenomena in near-critical water. Hicks spends much of his free time as a doctoral candidate at Case Western Reserve University putting the finishing touches on a dissertation he plans to complete in the fall of 2013.

combustion = particles
nice share..